How Many Elements In Glucose
What is Glucose?
Glucose is a elementary saccharide with six carbon atoms and one aldehyde group. This monosaccharide has a chemic formula C6H12Osix.
It is too known equally dextrose. It is referred to equally aldohexose as it contains 6 carbon atoms and an aldehyde grouping. Information technology exists in two forms, open-chain or band structure. It is synthesized in the liver and kidneys of animals. In plants, it is constitute in fruits and in unlike parts of plants. D- glucose is the naturally occurring form of glucose. It can occur either in solid or liquid form. It is water-soluble and is besides soluble in acetic acid. It is odourless and sweet to taste. In the yr 1747, Andreas Marggraf, a German chemist, isolated glucose from raisins. In the year 1838, Jean Baptiste Dumas coined the give-and-take glucose.
Table of Content
-
- Structure of Glucose
- Properties of Glucose
- Preparation of Glucose
- Uses of Glucose
- FAQs
Structure of Glucose – Chalf dozenH12Osix
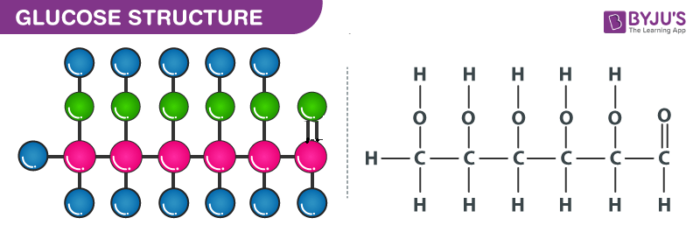
Properties of Glucose – C6H12O6
| C6H12Ovi | Glucose |
| Molecular Weight/ Molar Mass | 180.xvi g/mol |
| Density | 1.54 g/cm³ |
| Melting Bespeak | 146 °C |
| Unproblematic sugar | Monosaccharide |
Glucose can be called as aldohexose as well as dextrose. It is a monomer of many larger compounds such as carbohydrates, starch, and cellulose. On earth, this is the most abundant organic chemical compound. On the basis of the following prove, it was assigned the structure illustrated to a higher place:
- It has a molecular formula of C6H12Ohalf dozen
- When How-do-you-do is heated for a long time, north-hexane is formed which indicates that all the half dozen carbon atoms are linked in a directly chain.
- The oxime is formed when glucose reacts with hydroxylamine and cyanohydrins on the addition of hydrogen cyanide to it. This reaction can confirm the presence of the carbonyl group in glucose.
- On the reaction of glucose with a mild oxidizing agent like bromine water, the glucose gets oxidized to a carboxylic acid that contains 6 carbon atoms. This indicates that the carbonyl group is nowadays as an aldehyde grouping.
- The presence of -OH group is confirmed after the acetylation of glucose with acetic acid, which gives glucose pentaacetate.
- Glucose as well as gluconic acrid both yields dicarboxylic acid and saccharic acid on oxidation with nitric acrid. The presence of master alcohol is indicated by this.
Preparation Of Glucose (CviH12Osix)
Sucrose (cane saccharide) and starch are the ii major sources of Glucose.
Grooming from sucrose or cane carbohydrate:
Sucrose is a disaccharide with the formula C12H22O11. On boiling an aqueous solution of sucrose with dilute HCl or dilute H2SO4, Glucose and Fructose are formed in equimolar proportions.
C12H22O11 + H two O → CsixH12Ohalf dozen + Chalf dozenH12O6
Sucrose Glucose Fructose
Preparation from starch:
It is a polysaccharide that when boiled with dilute HtwoSOfour at 393 K nether ii to 3 atmosphere pressure, gives glucose.
( Chalf-dozenH12O5 ) n + n H ii O → nC6H12Ohalf dozen
Starch Glucose
Uses Of Glucose (C6H12O6)
- It is used in the treatment of hypoglycemia (low blood saccharide)
- It is given to patients who are very sick and cannot eat as information technology provides saccharide calories
- It is used in the treatment of increased potassium levels in the blood (hyperkalemia)
- Information technology is used every bit a precursor for the synthesis of substances.
Ofttimes Asked Questions- FAQs
How do you represent glucose?
The chemical formula of Glucose is CsixH12O6 . Glucose is a monosaccharide containing an aldehyde group (-CHO). Information technology is made of 6 carbon atoms, 12 hydrogen atoms and half dozen oxygen atoms. Glucose is an aldohexose.
Is glucose a reducing saccharide?
Glucose is a reducing sugar considering it belongs to the category of an aldose meaning its open-concatenation course contains an aldehyde grouping. By and large, an aldehyde is quite easily oxidized to carboxylic acids.
What are the five reducing sugars?
The five reducing sugars are ribose, glucose, galactose, glyceraldehyde, xylose.
What are the elements of glucose?
Glucose consists of three elements such as carbon, hydrogen and oxygen. 6 carbon atoms bonded together as a concatenation with additional atoms of oxygen and hydrogen.
What type of sugar is glucose?
Glucose is a monosaccharides sugar. Information technology occurs in the gratis state in the ripe grapes ( grape sugar) in dearest and also many sweet fruits. Glucose is an essential constituent of man blood which normally contains 65 mg to 110 mg of glucose per 100 mL. It is named equally blood sugar.
Too, Read:
Acquire more almost the isomerism, L-glucose, D- glucose and the construction of Chalf-dozenH12O6 from the expert faculties at BYJU'S.
How Many Elements In Glucose,
Source: https://byjus.com/chemistry/glucose/
Posted by: holterfatert94.blogspot.com


0 Response to "How Many Elements In Glucose"
Post a Comment